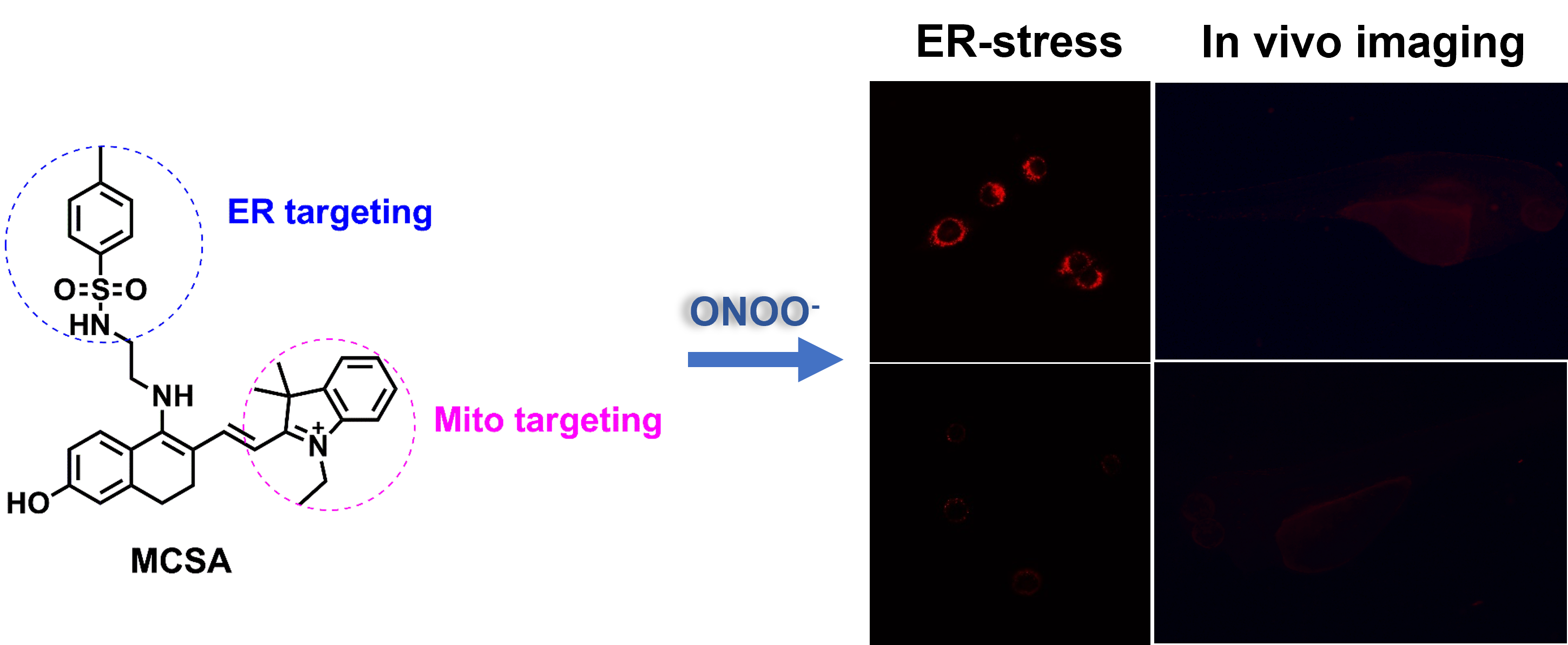
Figure 2 from Understanding the fate of peroxynitrite in plant cells--from physiology to pathophysiology.
Fig. 2. A hypothetical regulatory mechanisms of ONOO action in plant cells. In the biological milieu the peroxynitrite anion (ONOO ) is in equilibrium with peroxynitrous acid (ONOOH; pKa = 6.8). The reaction of ONOO with carbon dioxide leads to the formation of carbonate (CO 3 ) and nitrogen dioxide ( NO2) radicals. Alternatively, ONOOH can undergo homolytic fission to generate one-electron oxidants, hydroxyl OH and NO2 radicals. ONOOH readily crosses lipid bilayers and its decomposition to OH and NO2 radicals seems to become relevant in hydrophobic phases to initiate lipid peroxidation and lipid and protein nitration processes. If ONOO combines with SH-containing molecules (X-SH), it might be converted to S-nitroso compounds, e.g. S-nitrosoglutathione. Nitration and the formation of S-nitroso compounds are proposed mechanisms, by which ONOO regulates NO-dependent signaling events. On the other hand, nucleotides within DNA and RNA can undergo nitration by ONOO forming 8-nitroguanine, which may impart pathological consequences (Corpas et al., 2009b). - "Understanding the fate of peroxynitrite in plant cells--from physiology to pathophysiology."
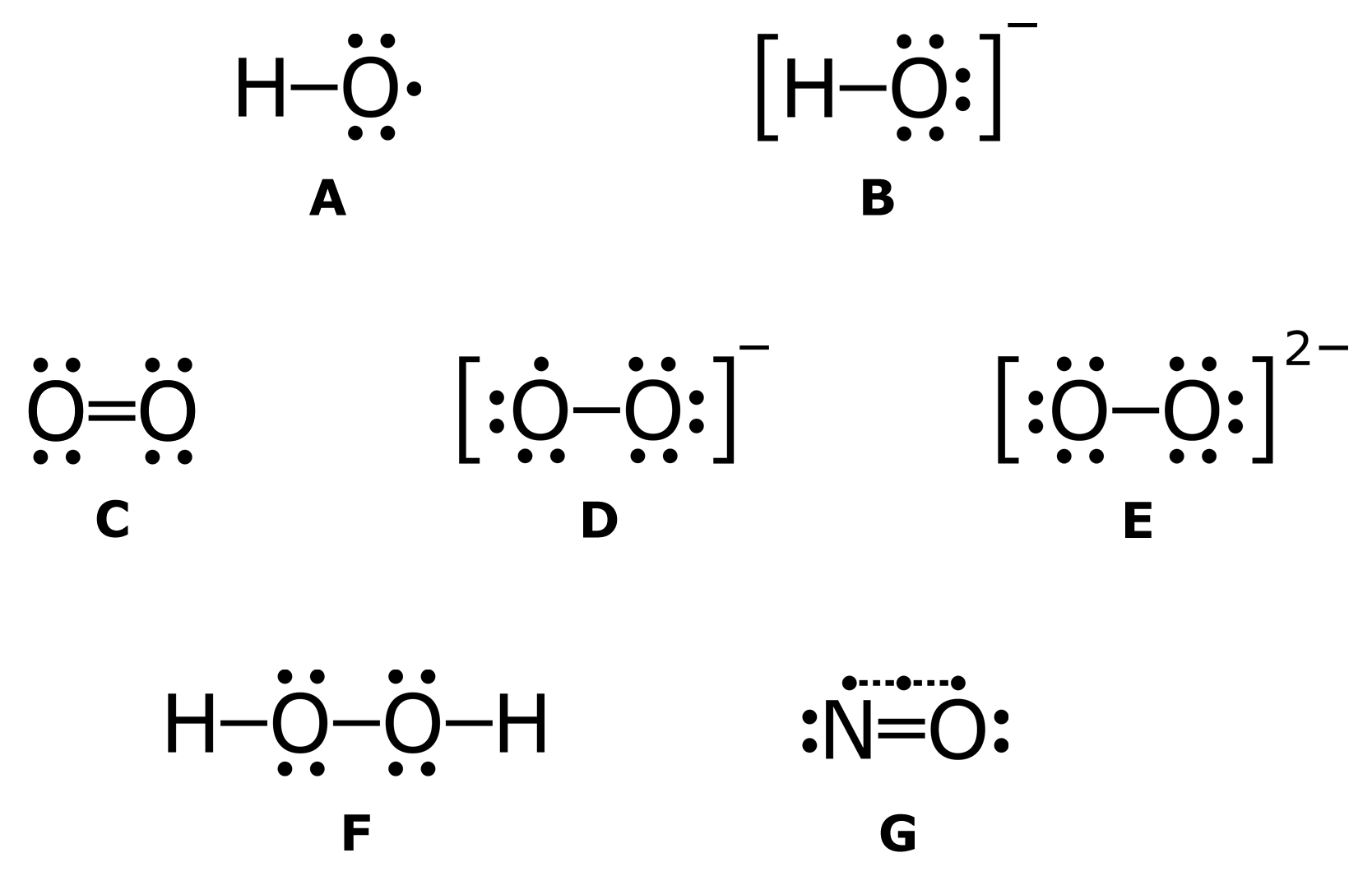
Reactive oxygen species - Wikipedia
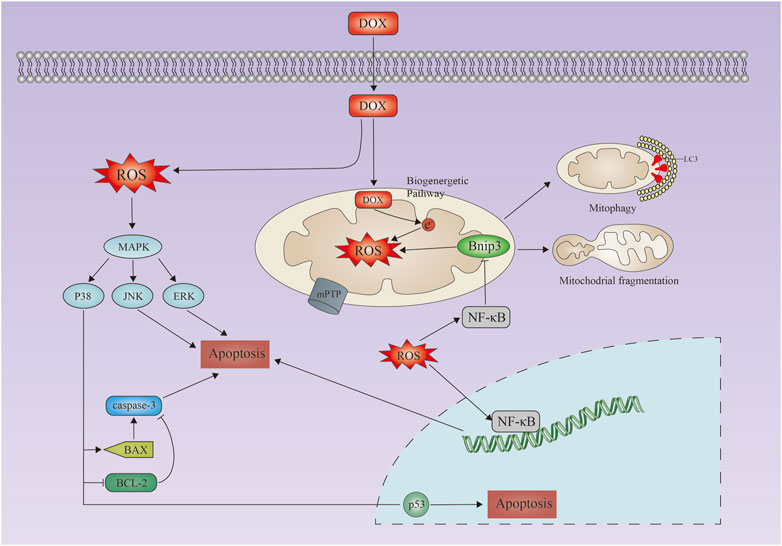
Frontiers Cell death regulation in myocardial toxicity induced

Journal of Cellular Physiology, Cell Biology Journal
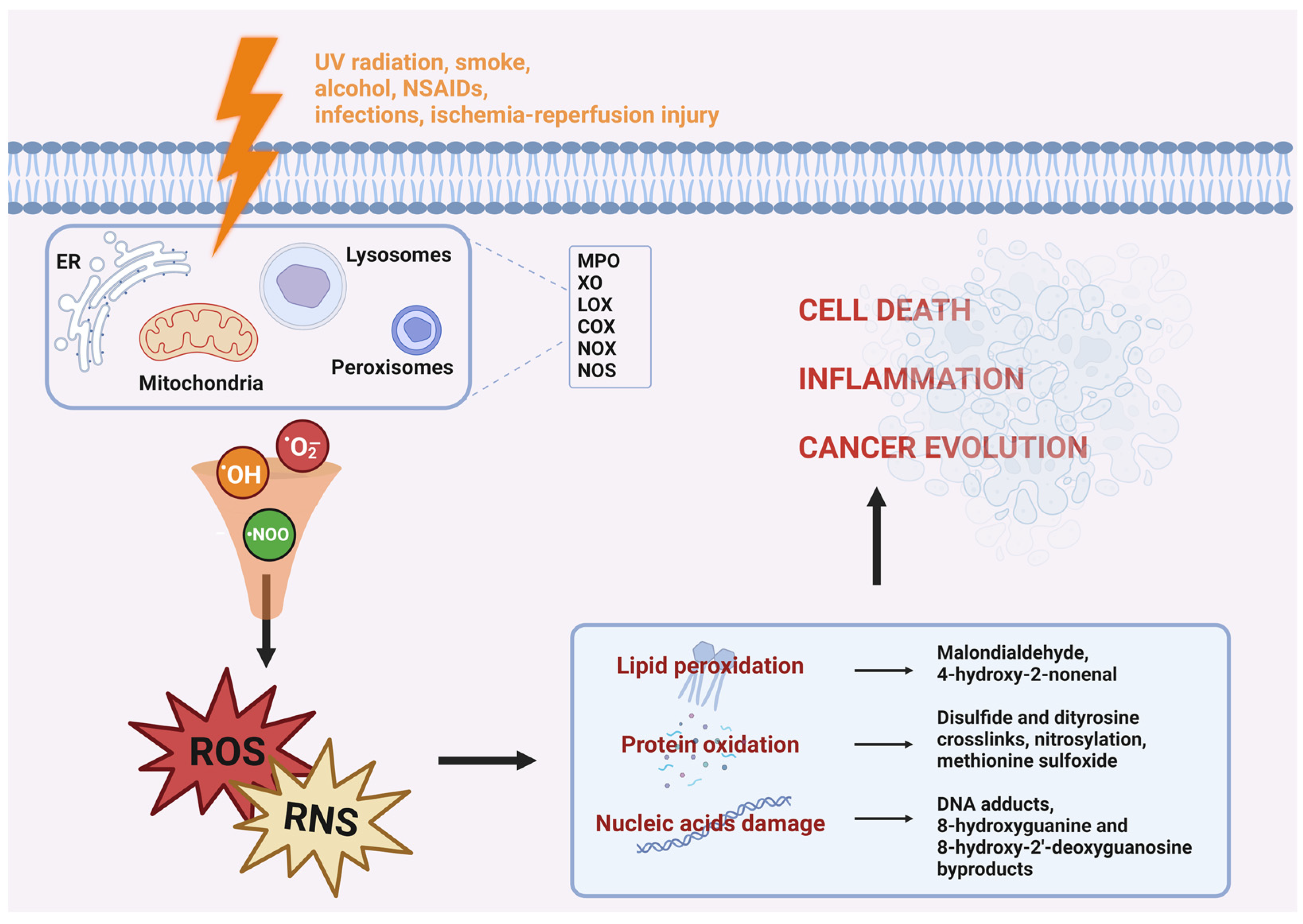
Oxygen, Free Full-Text

Association between the metabolome and bone mineral density in a

Chemical Biology of Peroxynitrite: Kinetics, Diffusion, and

Redox metabolism maintains the leukemogenic capacity and drug

Nitric oxide in plants: the history is just beginning - Beligni

Spontaneous Regression of Cancer: Revealing Granulocytes and
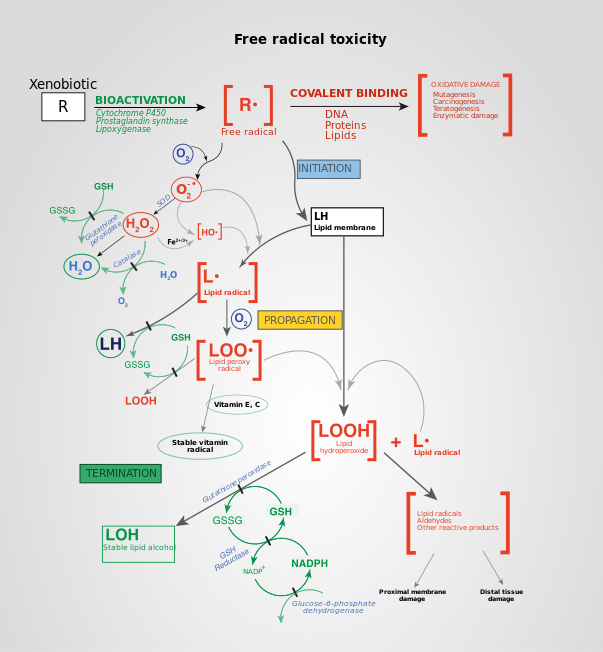
Reactive oxygen species - Wikipedia