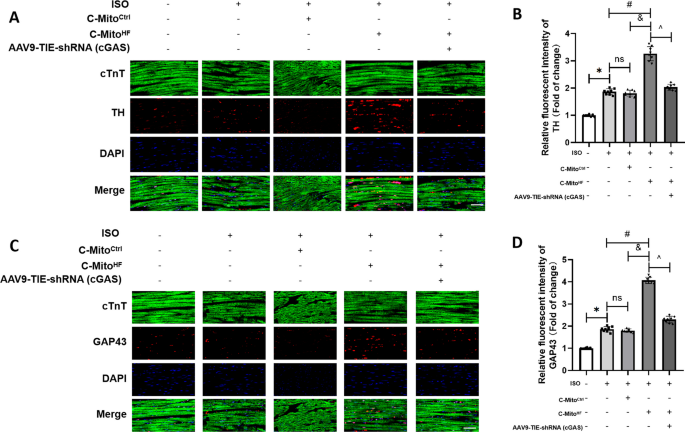
Circulating mitochondria promoted endothelial cGAS-derived neuroinflammation in subfornical organ to aggravate sympathetic overdrive in heart failure mice, Journal of Neuroinflammation

Figure S2. Experimental microglia depletion by PLX3397 (intragastric

PDF) Circulating mitochondria in organ donors promote allograft rejection

Quantitative comparison of echocardiographic parameters including
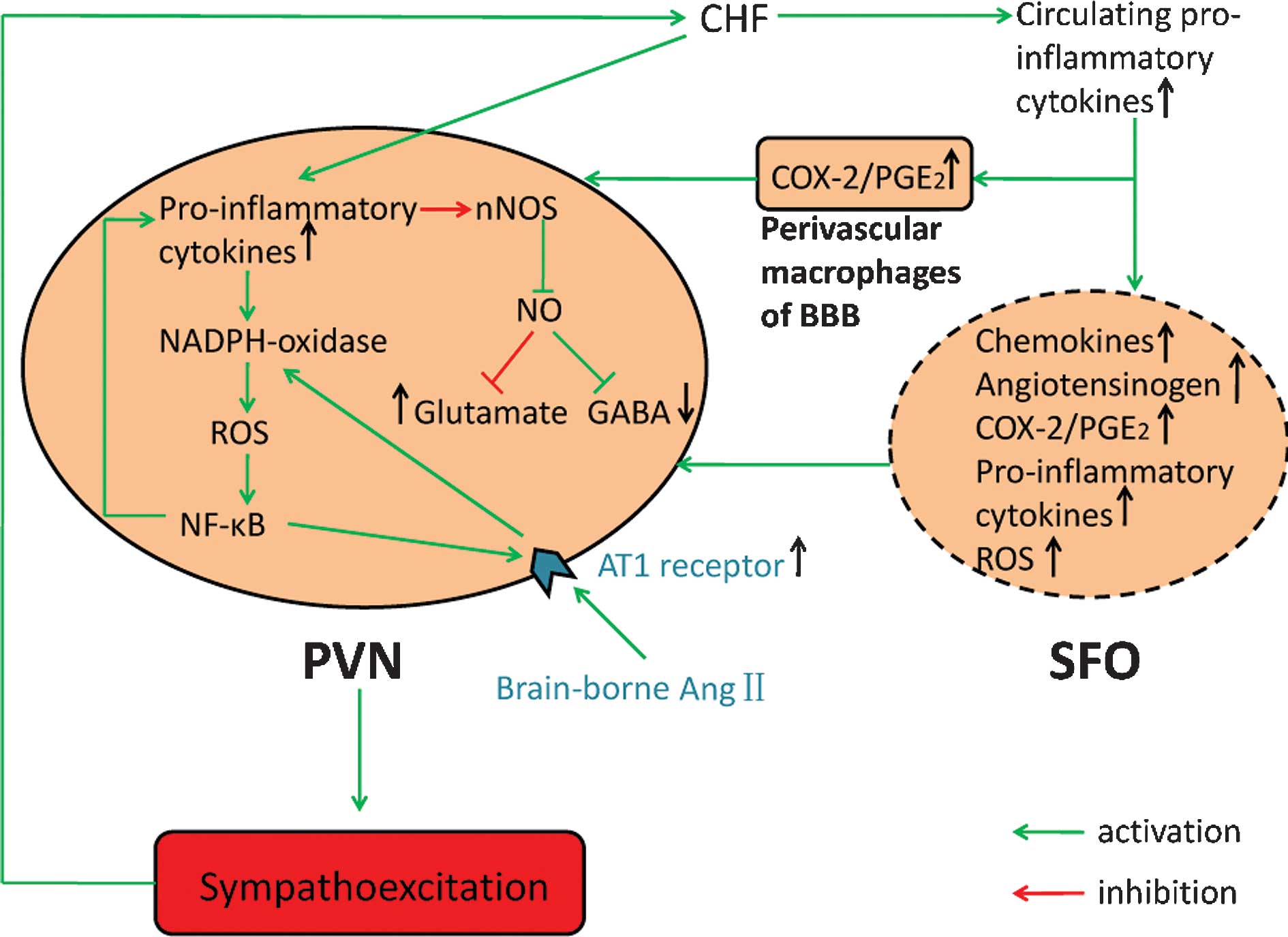
Brain mechanisms of sympathetic activation in heart failure: Roles of the renin‑angiotensin system, nitric oxide and pro‑inflammatory cytokines (Review)

Circulating mitochondria promoted endothelial cGAS-derived neuroinflammation in subfornical organ to aggravate sympathetic overdrive in heart failure mice

Frontiers Microglia-mediated inflammatory destruction of neuro- cardiovascular dysfunction after stroke

Circulating mitochondria promoted endothelial cGAS-derived neuroinflammation in subfornical organ to aggravate sympathetic overdrive in heart failure mice, Journal of Neuroinflammation
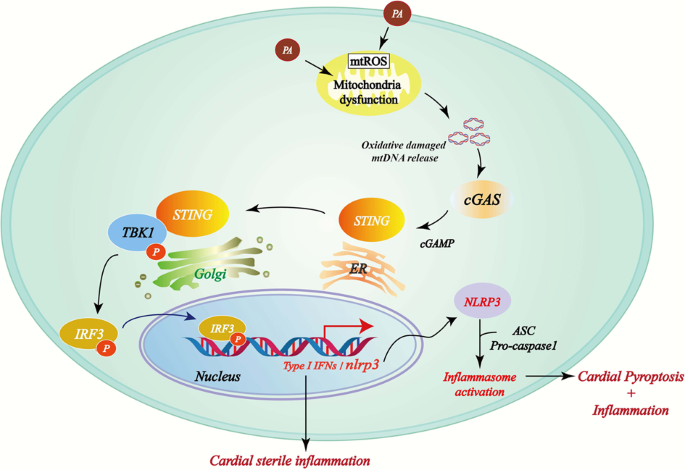
Mitochondrial damage and activation of the cytosolic DNA sensor cGAS–STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice

Clinical translation potential of mitoEVs. MitoEVs from injured or

Circulating mitochondria promoted endothelial cGAS-derived neuroinflammation in subfornical organ to aggravate sympathetic overdrive in heart failure mice, Journal of Neuroinflammation

Circulating mitochondria promoted endothelial cGAS-derived neuroinflammation in subfornical organ to aggravate sympathetic overdrive in heart failure mice, Journal of Neuroinflammation