
BNT162b2 Vaccine Booster and Mortality Due to Covid-19

Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5– and XBB/XBB.1.5–Related Sublineages Among Immunocompetent Adults — Increasing Community Access to Testing
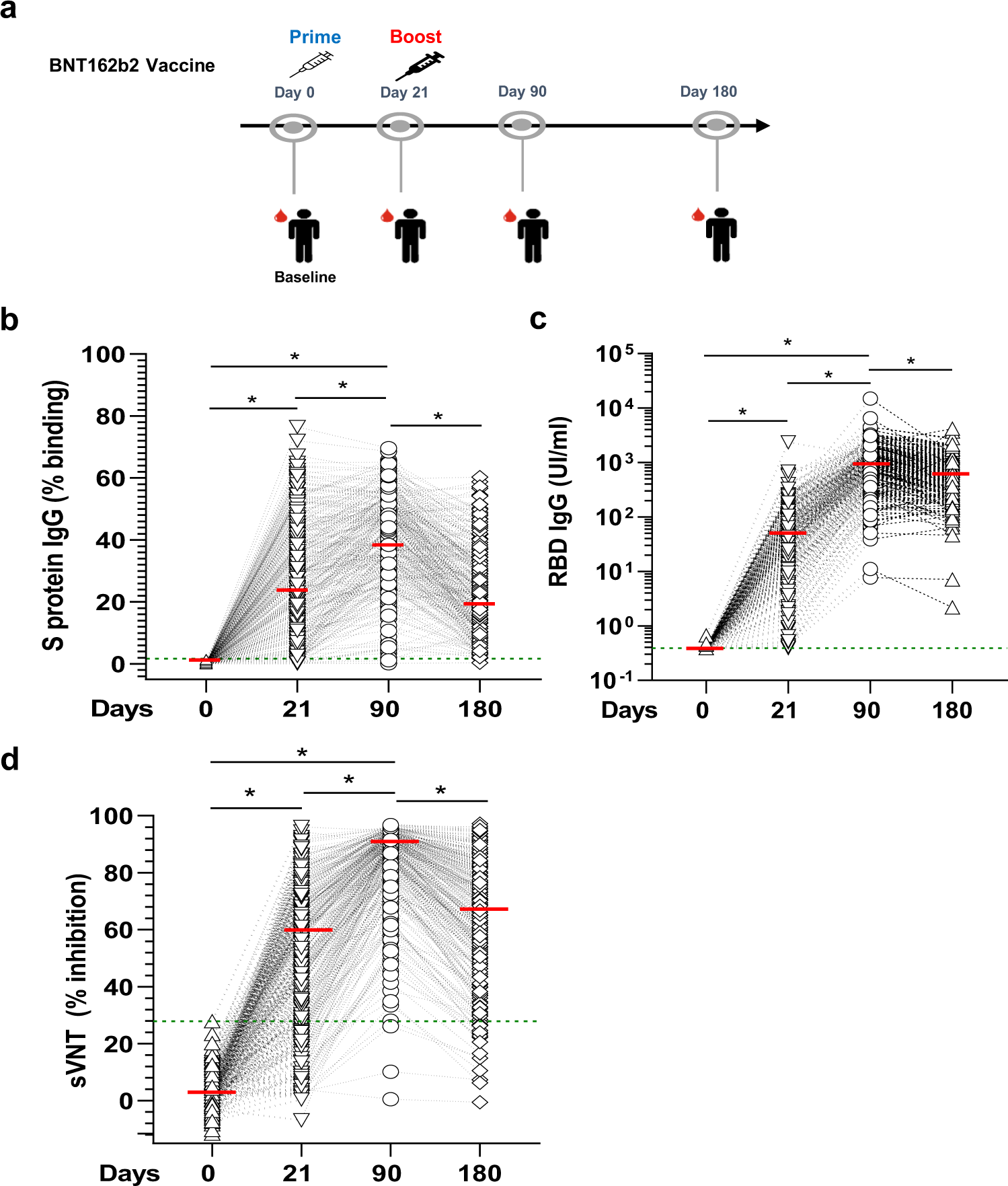
Lower vaccine-acquired immunity in the elderly population following two-dose BNT162b2 vaccination is alleviated by a third vaccine dose

Booster dose of BNT162b2 after two doses of CoronaVac improves neutralization of SARS-CoV-2 Omicron variant
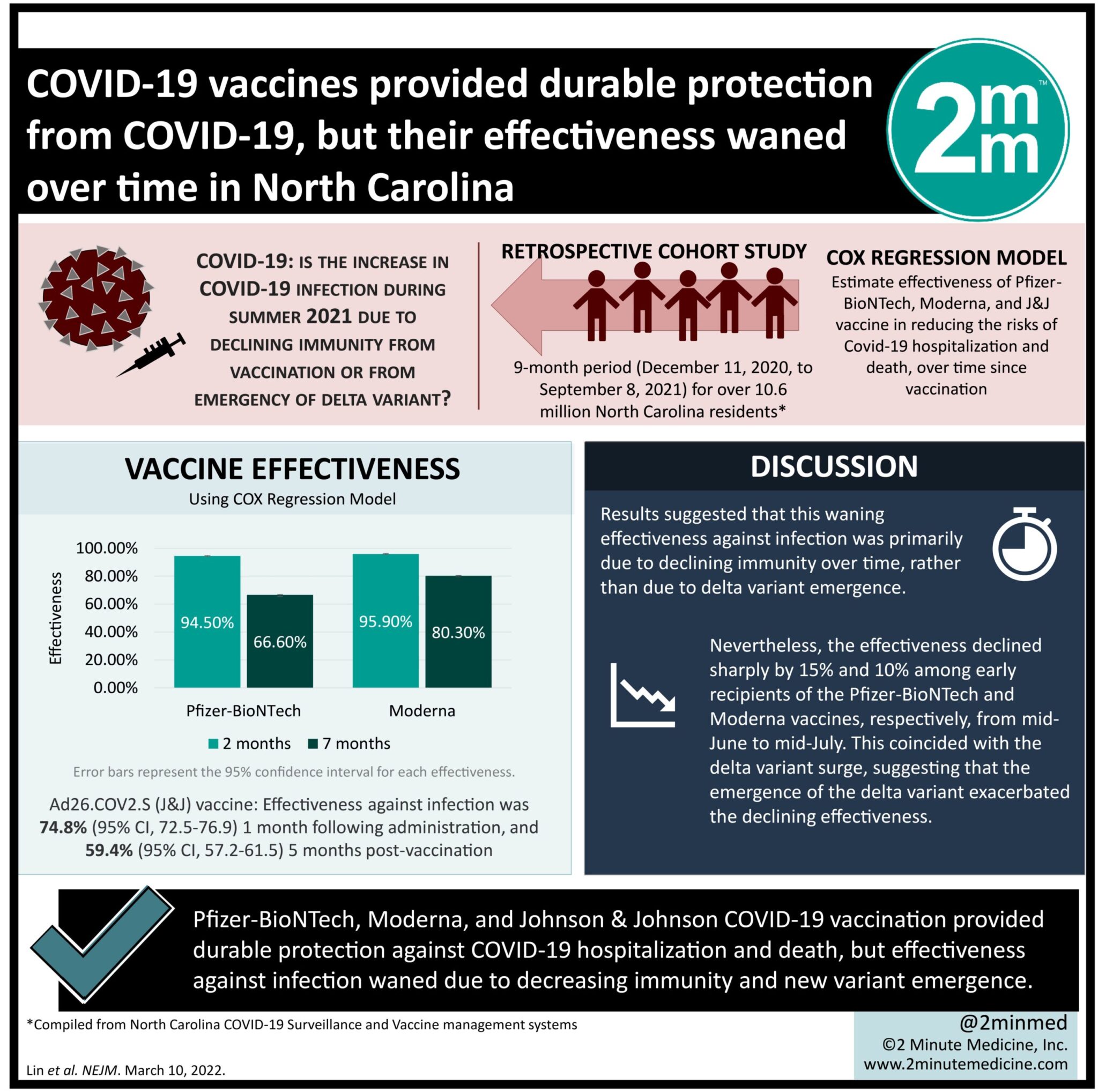
VisualAbstract: COVID-19 vaccines provided durable protection from COVID-19, but their effectiveness waned over time in North Carolina
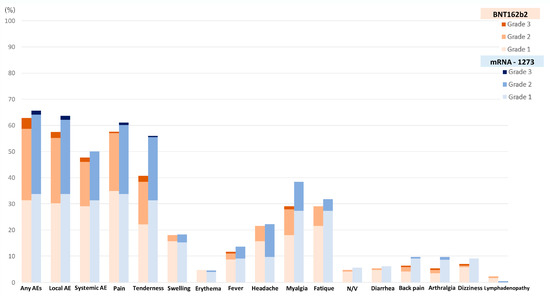
Vaccines, Free Full-Text

Effectiveness of BNT162b2 mRNA COVID-19 vaccine against SARS-CoV-2 variant Beta (B.1.351) among persons identified through contact tracing in Israel: A prospective cohort study - eClinicalMedicine
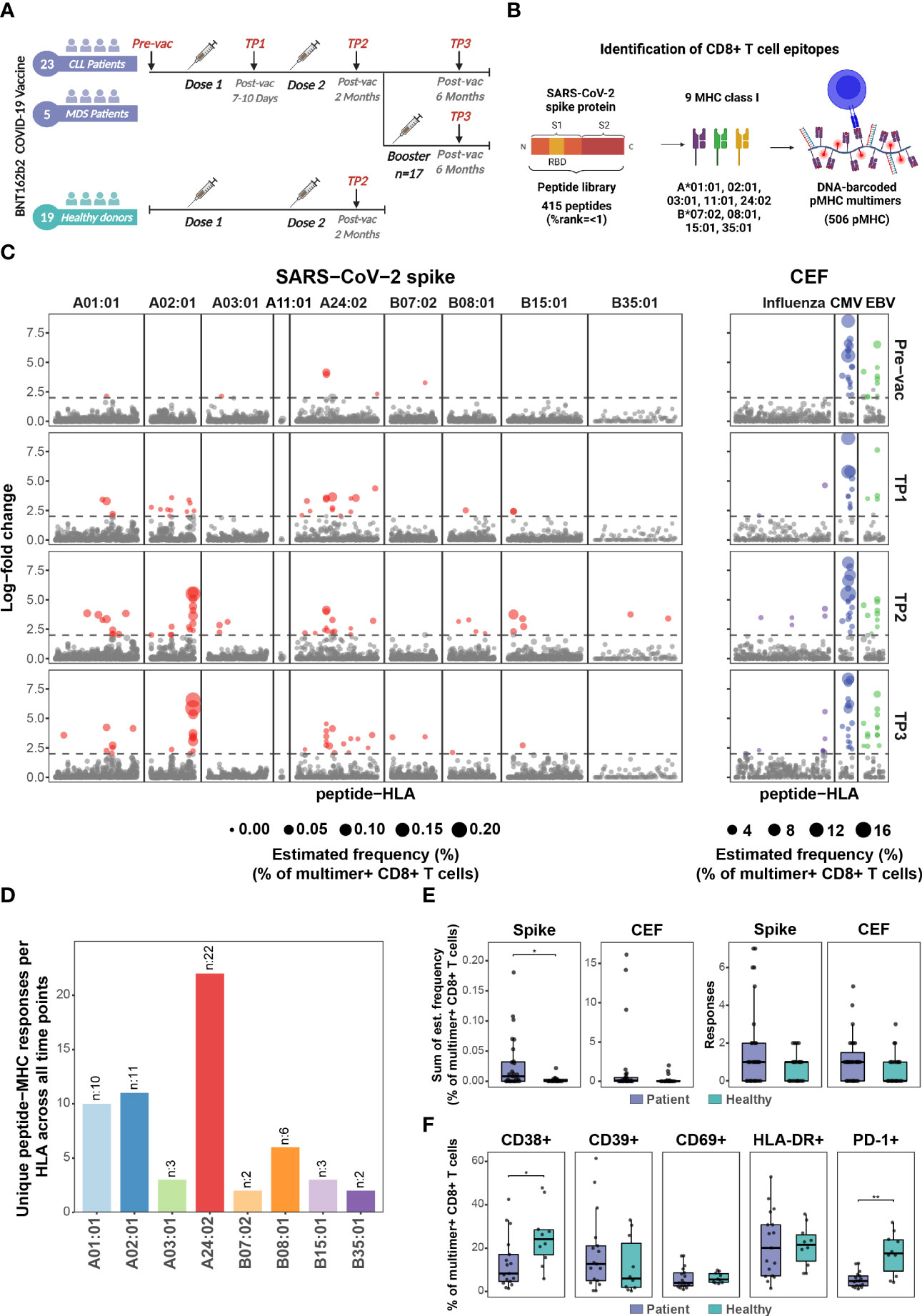
Frontiers Three doses of BNT162b2 COVID-19 mRNA vaccine establish long-lasting CD8+ T cell immunity in CLL and MDS patients

Third vaccine dose reduces transmission and severe COVID-19 nationwide in Israel

Effects of BNT162b2 Covid-19 Vaccine Booster in Long-Term Care Facilities in Israel

Vaccines, Free Full-Text

JCI Insight - Consecutive BNT162b2 mRNA vaccination induces short-term epigenetic memory in innate immune cells

Safety and Efficacy of a Third Dose of BNT162b2 Covid-19 Vaccine




,type=downsize,aspect=fit;Crop,size=(450,450),gravity=Center,allowExpansion;BackgroundColor,color=ffffff;UnsharpMask,gain=1.0,threshold=0.05;)




