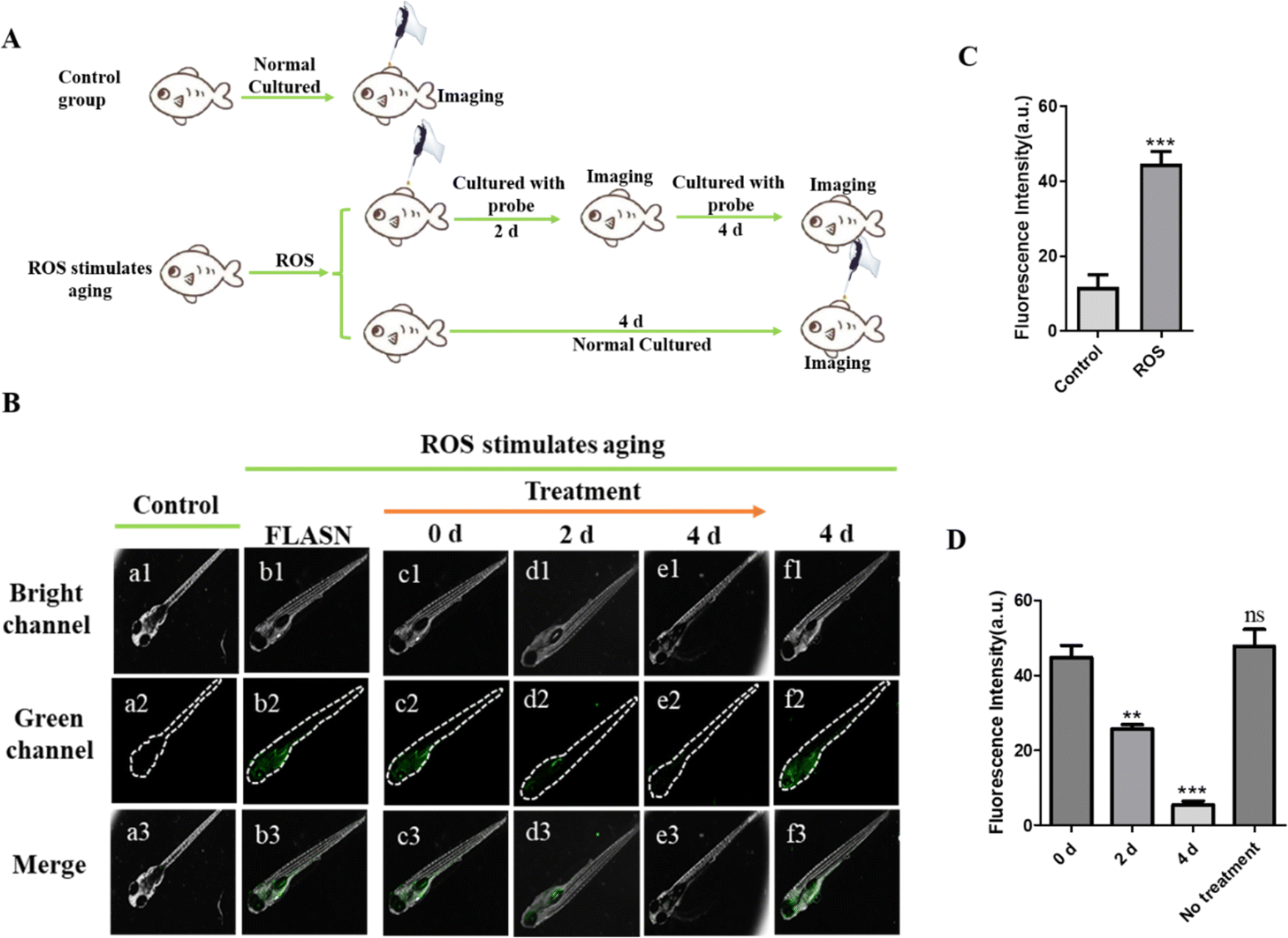
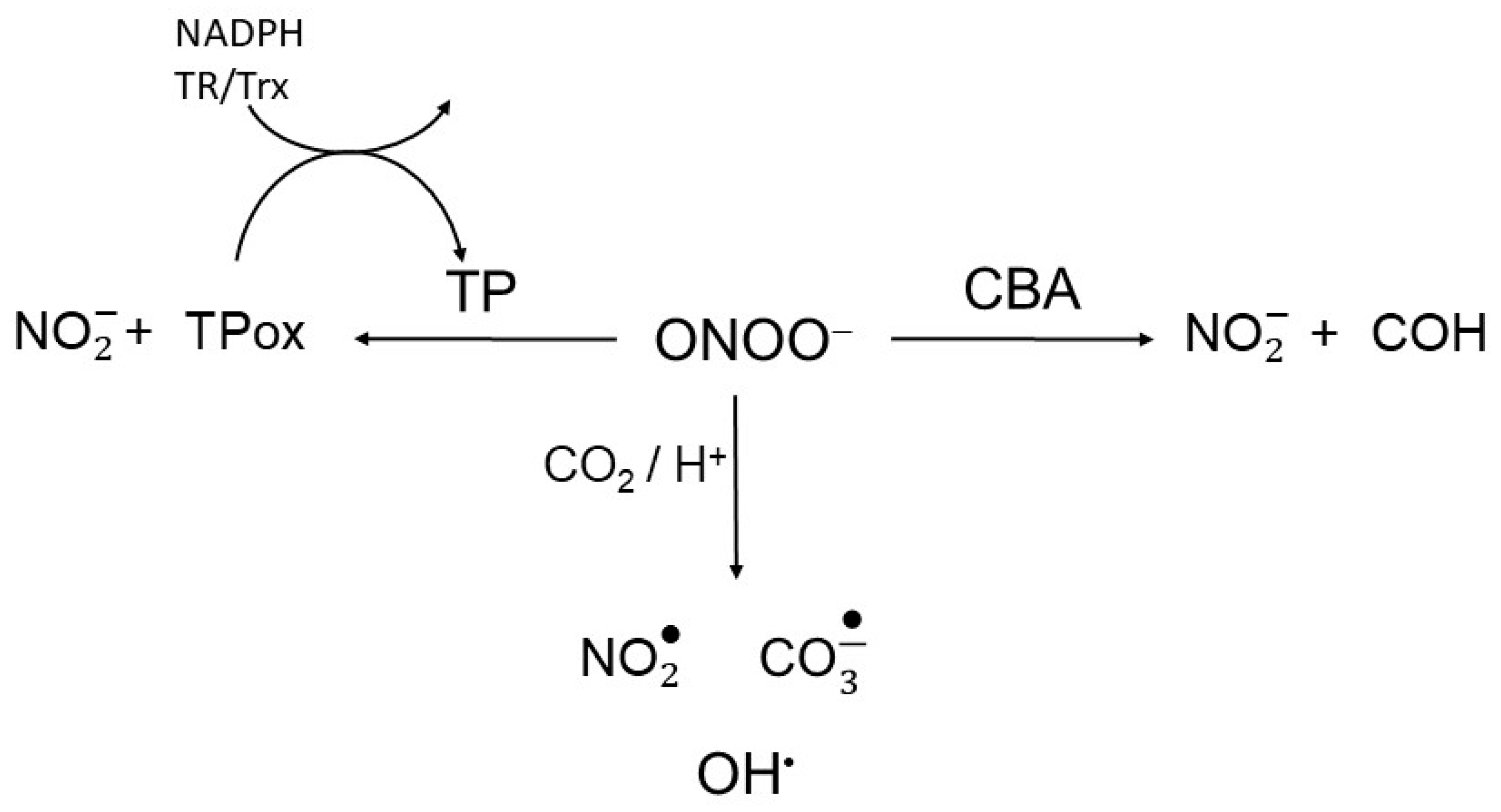
The species H2O2, ONOO⁻, NO, NO2⁻ being directly oxidized at their
Download scientific diagram | The species H2O2, ONOO⁻, NO, NO2⁻ being directly oxidized at their distinct potentials and their reconstructed fluxes. Reproduced from [2] with permission from Wiley from publication: Nanomaterial-based electrochemical sensors and optical probes for detection and imaging of peroxynitrite: a review | Peroxynitrite (PON for short) is a powerful nitrating, nitrosating and oxidative agent for cellular constituents. In vivo, PON is formed through the diffusion-controlled reaction between superoxide radical (O2•-) and nitric oxide (•NO). This critical review (with 67 refs.) | Electrochemical Sensors, Theranostics and Biocompatibility | ResearchGate, the professional network for scientists.
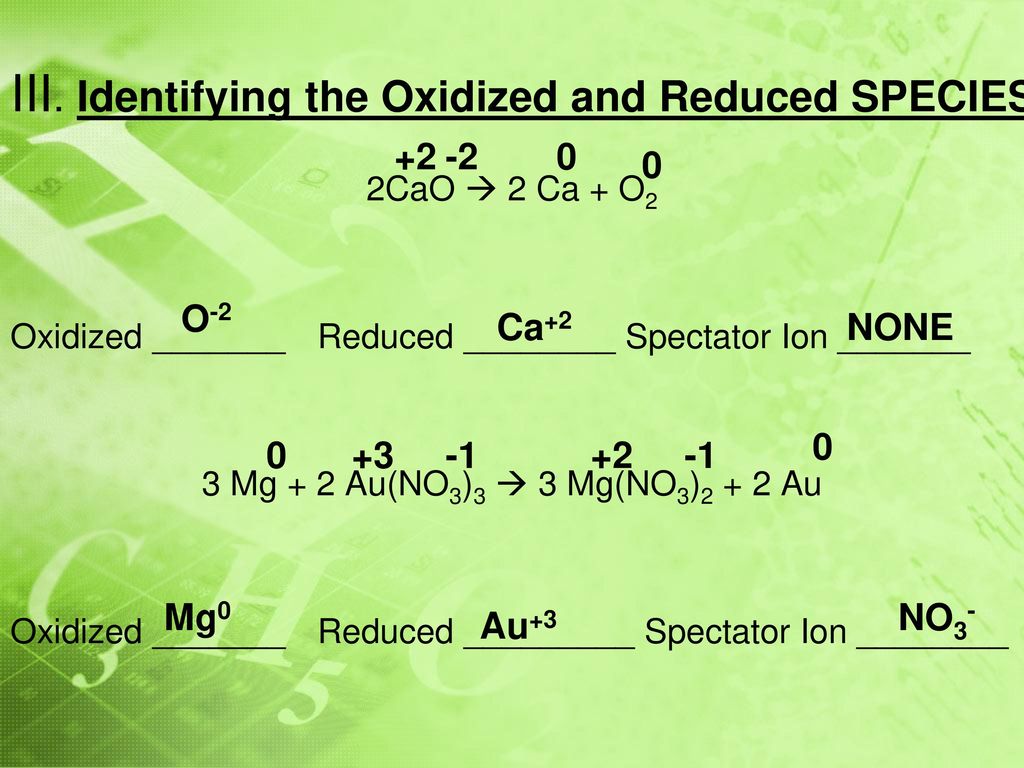
Unit 9: Oxidation, Reduction, and Electrochemistry - ppt download

Role of reactive nitrogen species in fatty acid nitration

Biochem Exam 2 Lecture 1 Flashcards

Targeting the NO/superoxide ratio in adipose tissue: relevance to
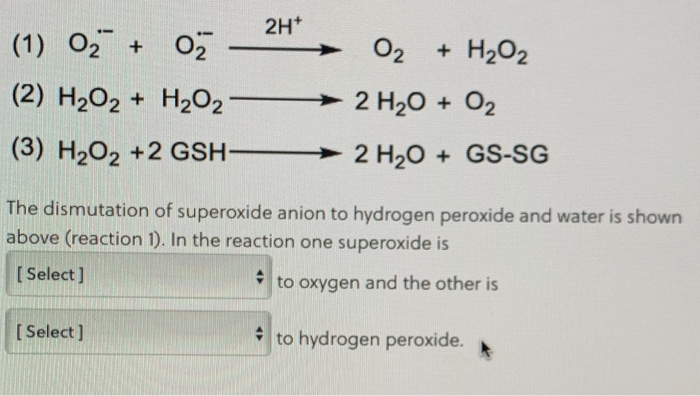
Solved please explain superoxide is reduced or oxidized?and

Guideline for screening antioxidant against lipid‐peroxidation by

Water-soluble cationic boronate probe based on coumarin

PDF) The reaction of H2O2 with NO2 and NO

NO Oxidation Using H2O2 at a Single-Atom Iron Catalyst

H2O2 acts only as an oxidising agent. H2O2⟶H2O + O