
Medicaid coverage practices for approved gene and cell therapies: Existing barriers and proposed policy solutions: Molecular Therapy Methods & Clinical Development

CMS and outcomes-based pricing for cell and gene therapy
Disparities in Clinical Research and Cancer Treatment

PDF) Medicaid Coverage Practices for Approved Gene and Cell Therapies: Existing Barriers and Proposed Policy Solutions

The EveryLife Foundation submits comments to the Centers for Medicare and Medicaid Services (CMS) on the Physician Fee Schedule Proposed Rule for 2021 - EveryLife Foundation for Rare Diseases
7 Developing and Delivering the Next Generation of Therapies, Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action

Bridging Gaps for Affordable Cell and Gene Therapies: Overcoming Financial and Systematic Obstacles
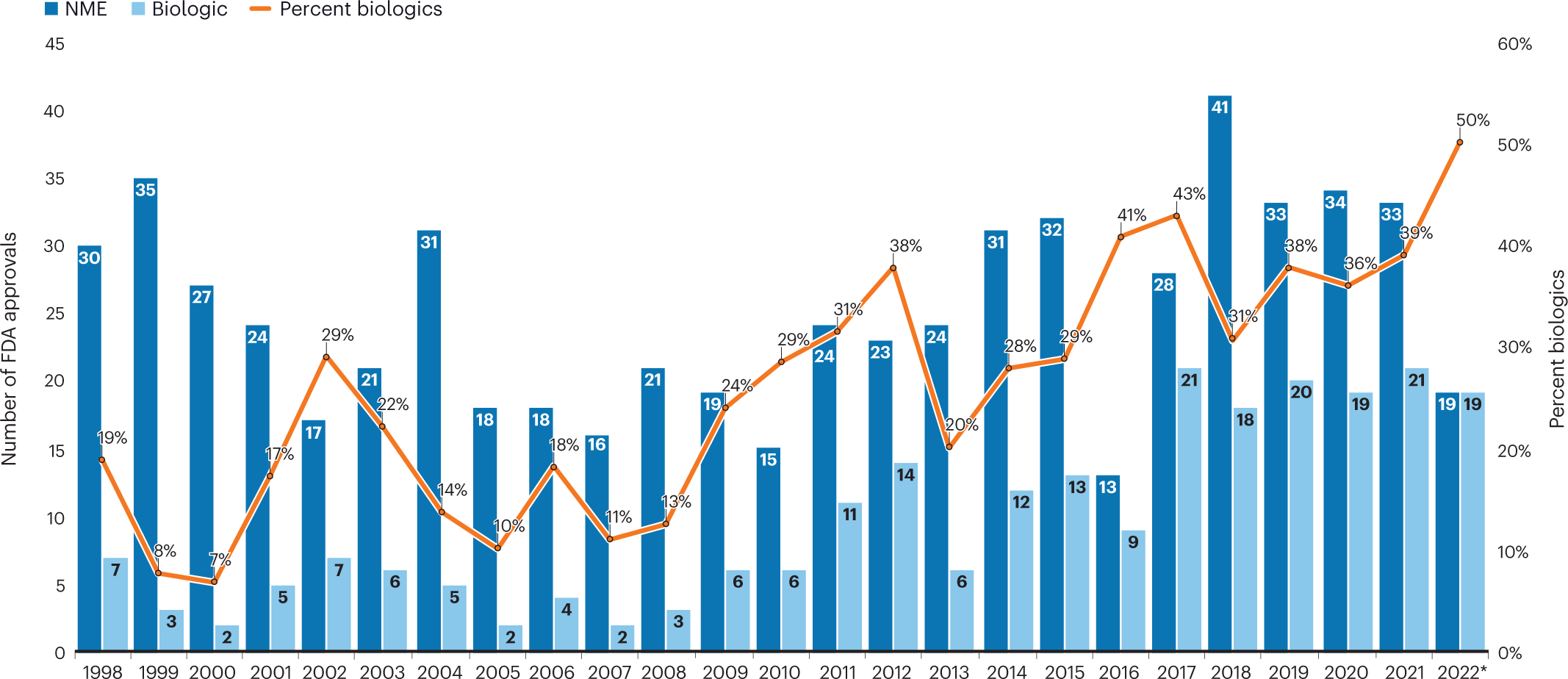
Fresh from the biotech pipeline: fewer approvals, but biologics gain share
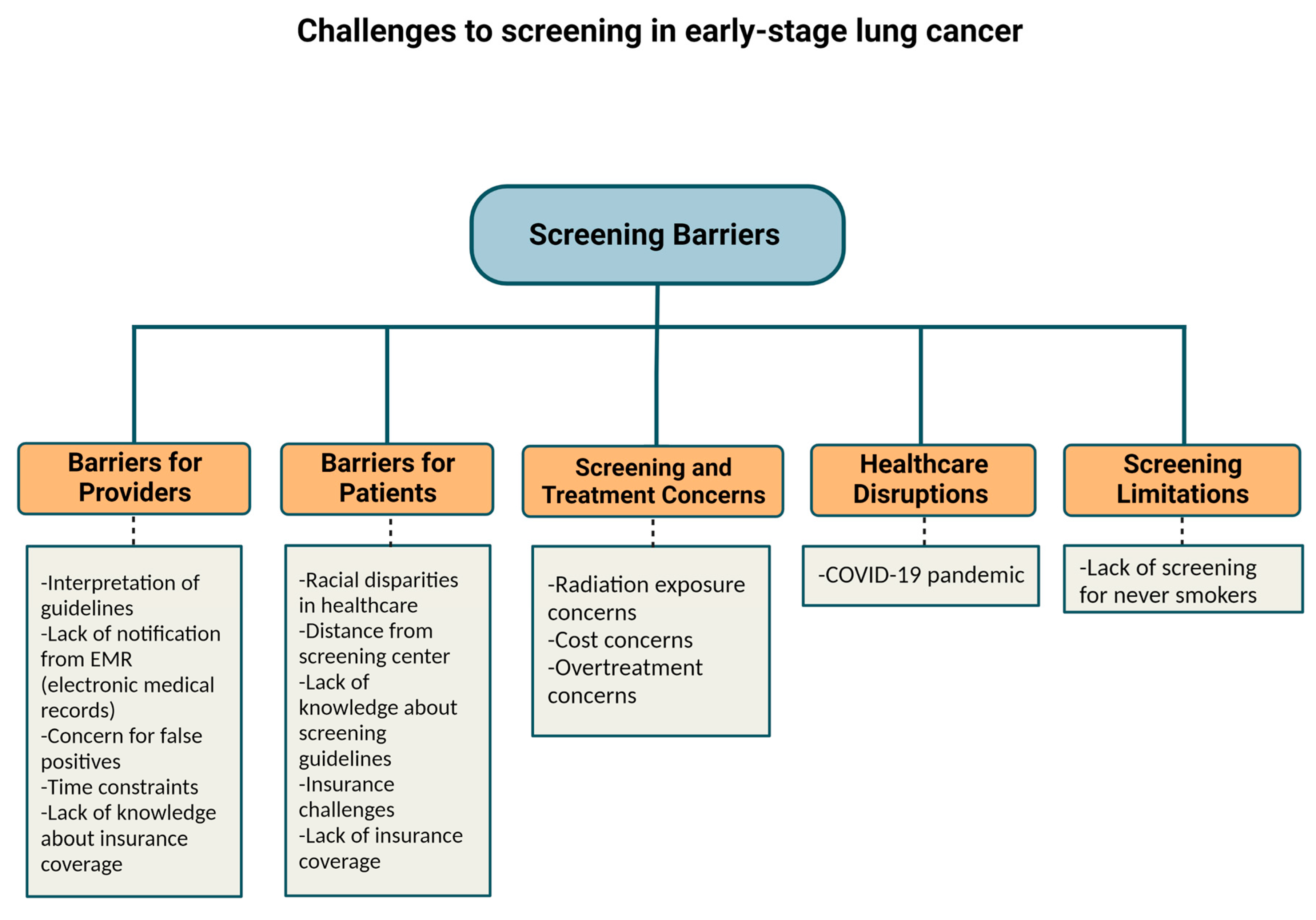
Cancers, Free Full-Text

Bridging Gaps for Affordable Cell and Gene Therapies: Overcoming Financial and Systematic Obstacles
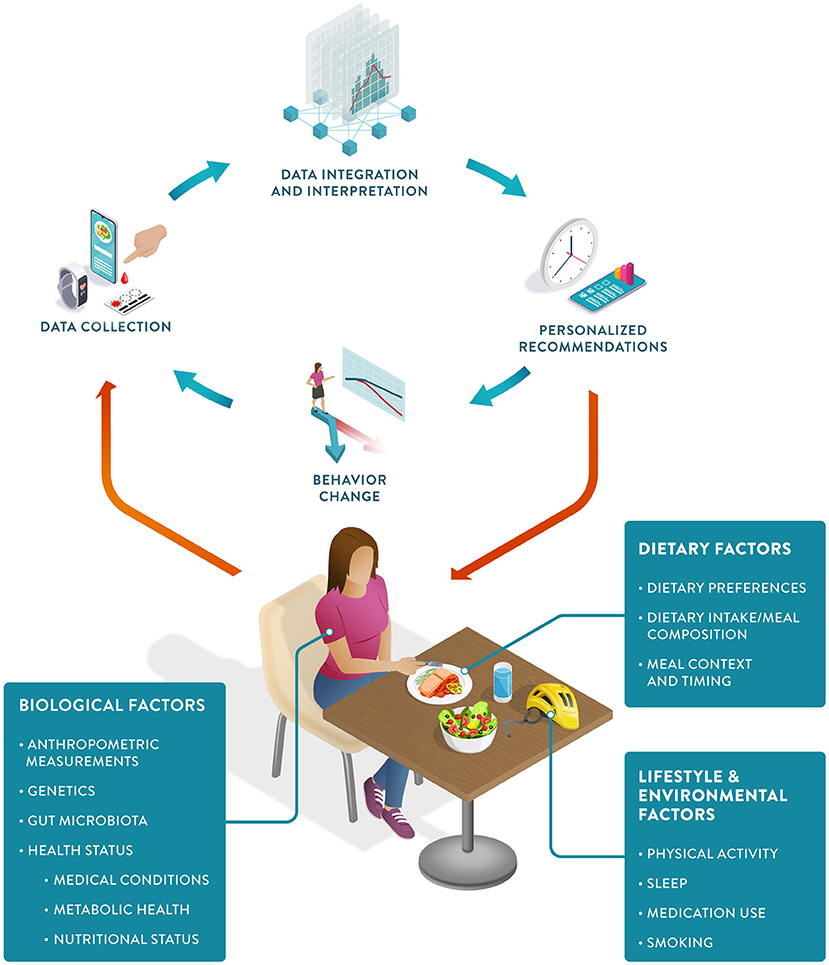
Frontiers Precision nutrition: Maintaining scientific integrity while realizing market potential
3 Supportive Policy Environment for Biomarker Tests for Molecularly Targeted Therapies, Biomarker Tests for Molecularly Targeted Therapies: Key to Unlocking Precision Medicine

Researchers Highlight Restrictive Coverage of Gene, Cell Therapies
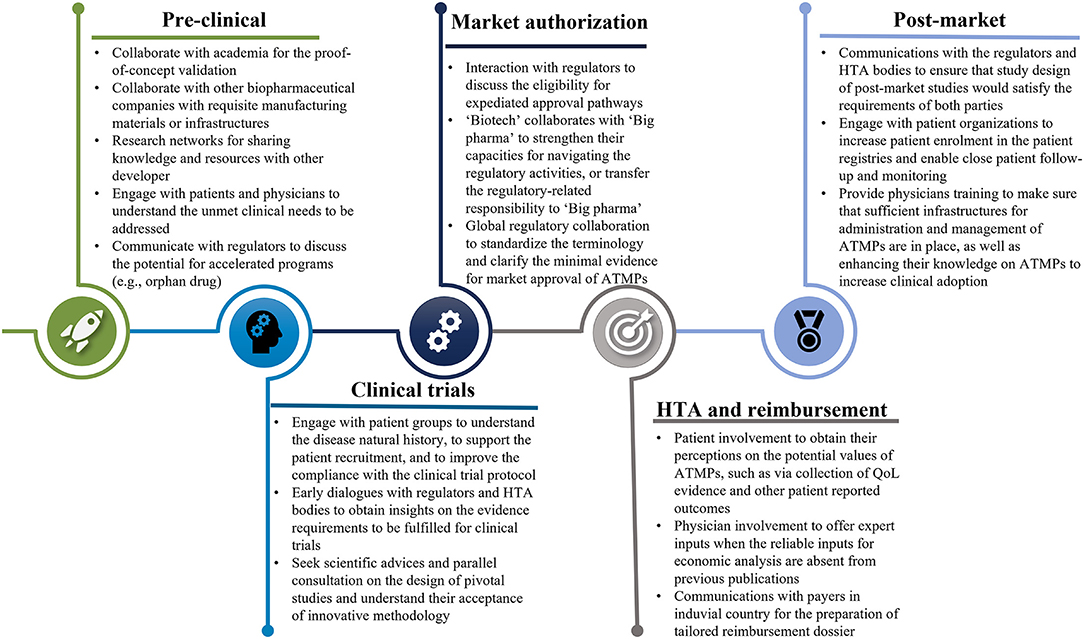
Frontiers Reinforcing Collaboration and Harmonization to Unlock the Potentials of Advanced Therapy Medical Products: Future Efforts Are Awaited From Manufacturers and Decision-Makers









