
FDA Says IQOS Tobacco Products Reduce Exposure to Harmful

Milestone' decision as FDA authorises IQOS as Modified Risk

Heated Tobacco Products at the Point of Sale – Counter Tobacco

FDA authorizes 'modified risk' marketing for IQOS system – Counter

FDA Authorizes Marketing of IQOS Tobacco Heating System With

US FDA authorizes marketing of IQOS as a Modified Risk Tobacco Product

It's Not a Cigarette. It's Not a Vape. And It's Big in Japan.
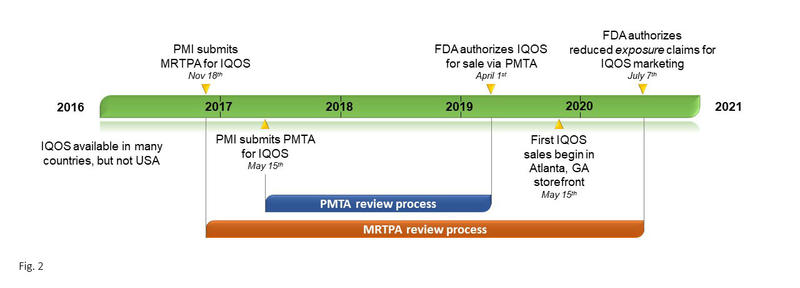
Controversy Regarding U.S. Marketing of New Heated Tobacco Product
Philip Morris device knows a lot about your smoking habit

Philip Morris Allowed to Say IQOS Reduces Harmful Exposure - Bloomberg

Tobacco Heating System (THS)

IQOS in the U.S.
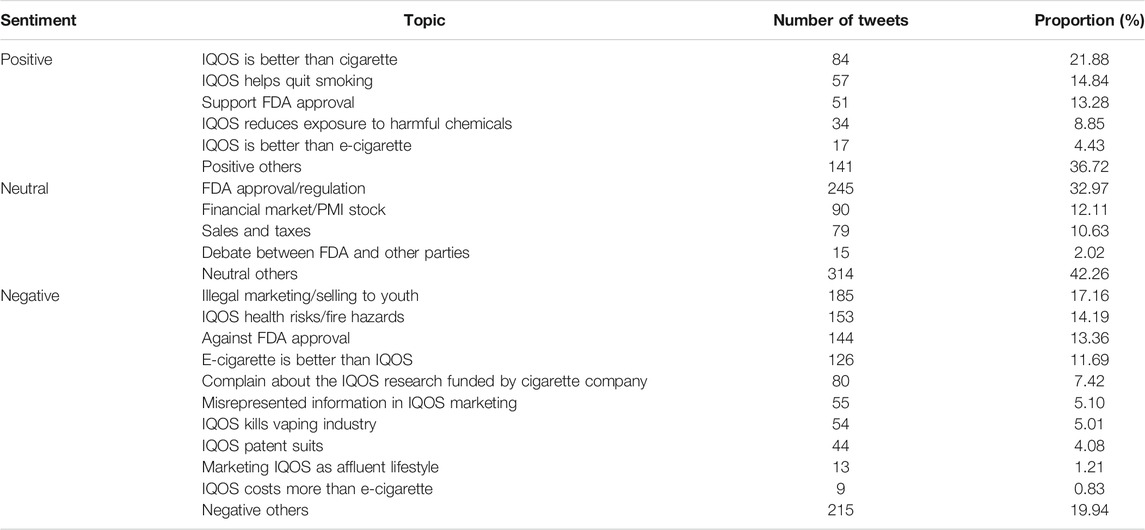
Frontiers Perceptions of the IQOS Heated Tobacco Product on

Tobacco Truth: FDA Advisory Committee: Heat-Not-Burn is Lower

Milestone' decision as FDA authorises IQOS as Modified Risk









